Biochemistry & Bioanalytical
Biomarker Detection
GD3 specializes in delivering tailored protein and nucleic acid biomarker assays to accelerate drug discovery and development. With a deep focus on immunology, ophthalmology, inflammation, infectious disease, oncology, CNS, and metabolic disease, we provide both custom and off-the-shelf solutions.
Beyond standard human, mouse, rat, and non-human primate assays, our in-house capabilities enable us to develop custom biomarkers for a wide range of species. This unique strength empowers researchers to translate proteomics findings into actionable clinical insights, driving medical breakthroughs.
-
Biomarker detection methods include
- Meso scale discovery and electrochemiluminescence (ECL)-based immunoassays
- Colorimetric, luminescence and fluorescent-based ELISAs
- Custom and commercially available immunoassays & ligand binding assays for analysis of protein biomarkers – either endogenous or your therapeutic agent
- Protein-protein interactions and protein-DNA interactions
- Reporter bioassays and cell signaling assays
- Protein purity and aggregation analysis
- Real-time PCR analysis
- Differential gene expression via RNAseq
- Western blots
- Anti-drug antibody assays
- Cytokine Panels
- Biomarker Discovery
-
Biomarker examples include:
- Metabolic Markers
- Oncology Markers
- Vascular Markers
- Cytokines and Chemokines
- Cell-Signaling Pathways
- Alzheimer’s (Aβ38, Aβ40, Aβ42, Tau, Total Tau)
- Kidney Injury
- Cardiac and Muscle
- Liver Injury
- Inflammation
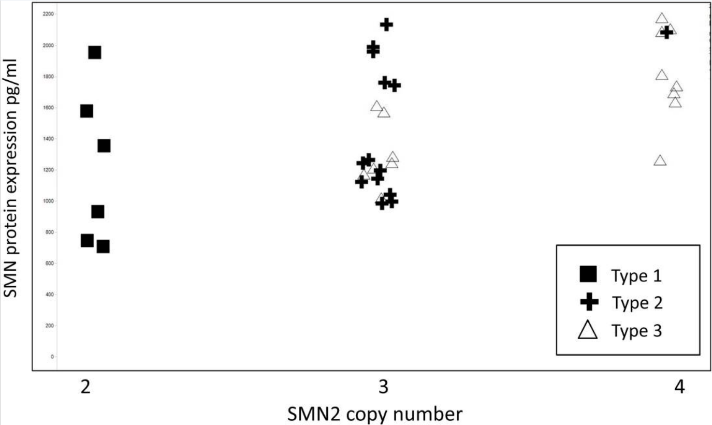
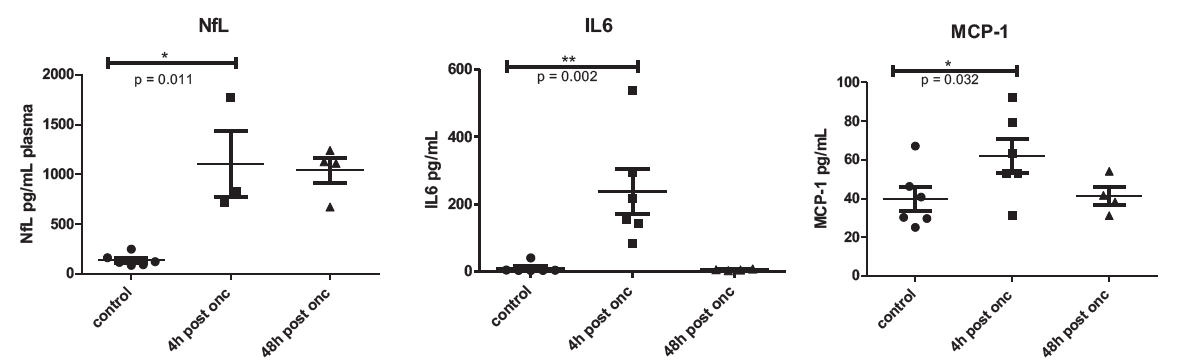
Learn more about Ocular Efficacy Models






























