Biochemistry & Bioanalytical
Allergic Conjunctivitis
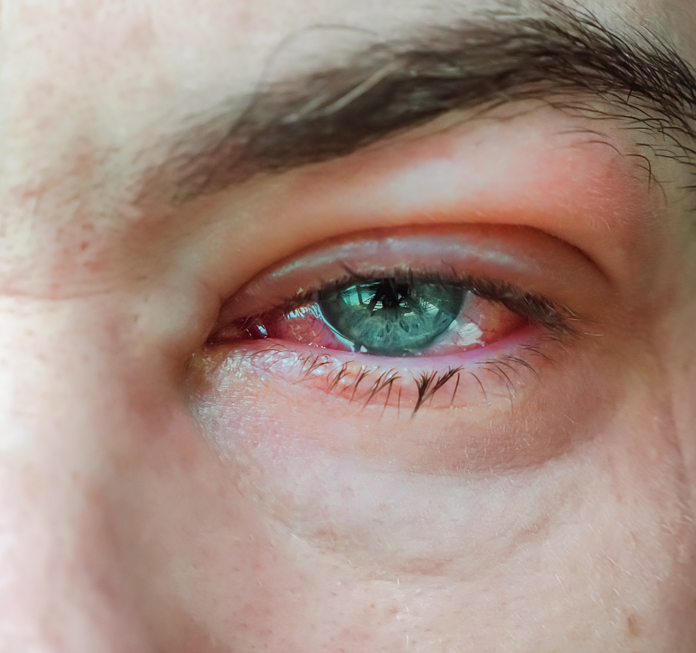
Allergic conjunctivitis is an increasingly prevalent ocular allergy and is classified as a hypersensitivity disorder that affects the lid, conjunctiva, and cornea1. Mast cells are highly populated in the choroid, subconjunctival and episcleral tissues, and the degranulation of these cells is largely responsible for the immediate hypersensitivity reactions seen in allergic conjunctivitis disease pathology2.
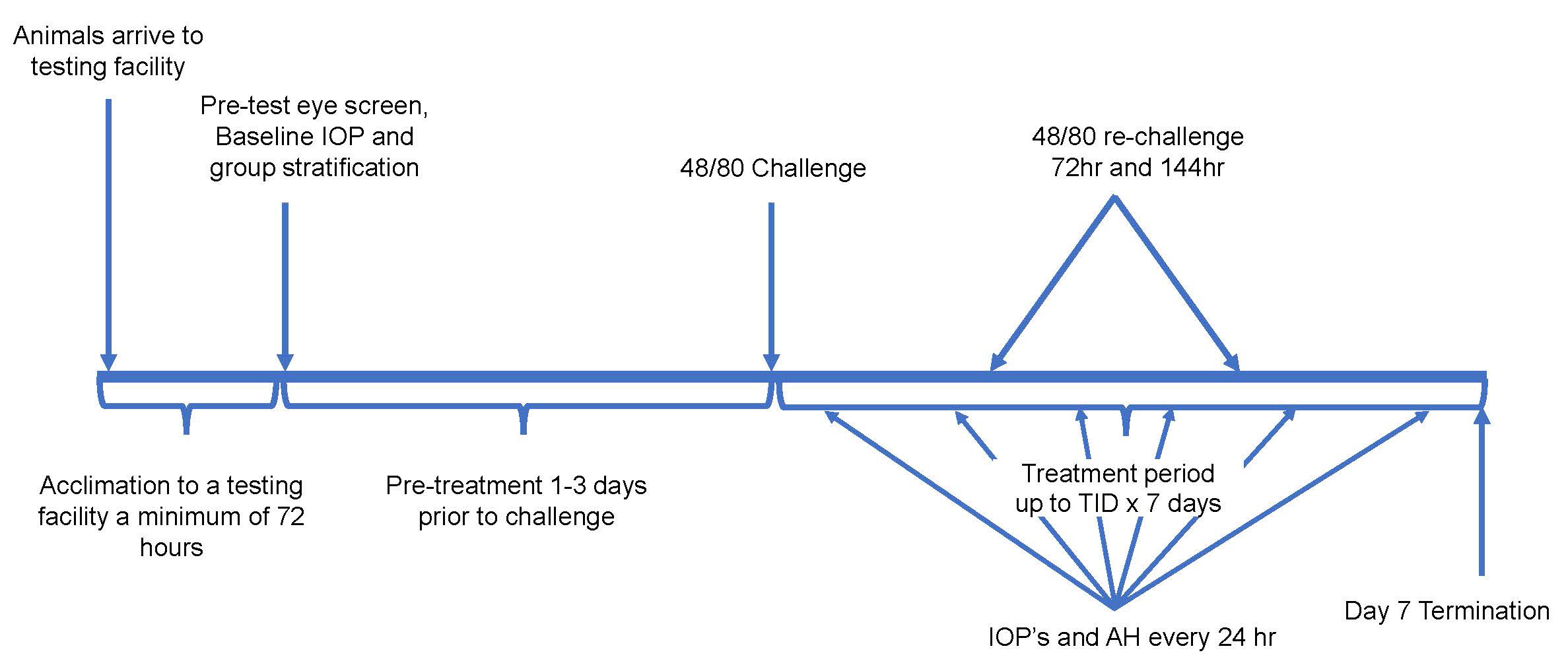
The experts at GD3 replicate this disease pathology through topical administration of Compound 48/80, which is a mast cell degranulating agent3. Following successful induction, we are enabled to confirm and longitudinally follow disease status and understand the effect that various types of therapeutic candidates have on disease pathology.
| Animal Species | Rabbit |
| Method of Induction | Topical administration of Compound 48/80 |
| Follow up Period | 24 hrs. to 1 week |
| Route of intervention | Topical (ocular), IVT, systemic |
| Readouts |
|
Reference:
- 1. Benditt and Lagunoff, 1964
- 2. Hogan, Alvarado and Weddell, 1971).
- 3. https://pubmed.ncbi.nlm.nih.gov/6838424/
Learn more about





































