Disease Modeling
Patient-derived xenografts (PDX) are models of cancer where the tissue or cells from a patient's tumor are implanted directly into an animal model. PDX models are important preclinical tools for developing effective and safe therapeutics that combat cancer. Drug developers can utilize PDX models to target patient tumors with specific oncogene profiles. PDX models are significantly more accurate in predicting clinical efficacy than other, more conventional approaches. This predictive accuracy helps our clients develop successful drug candidates by providing a more accurate assessment of therapeutic efficacy, thereby enabling better decision-making and allocation of time and resources.
Please register or login to view detailed model informationWith GD3 clients receive a boutique experience with highly skilled experts customized for their study.
Is this right for you? Contact us to speak with one of our experts to start building your study.
Request a QuoteLearn more about
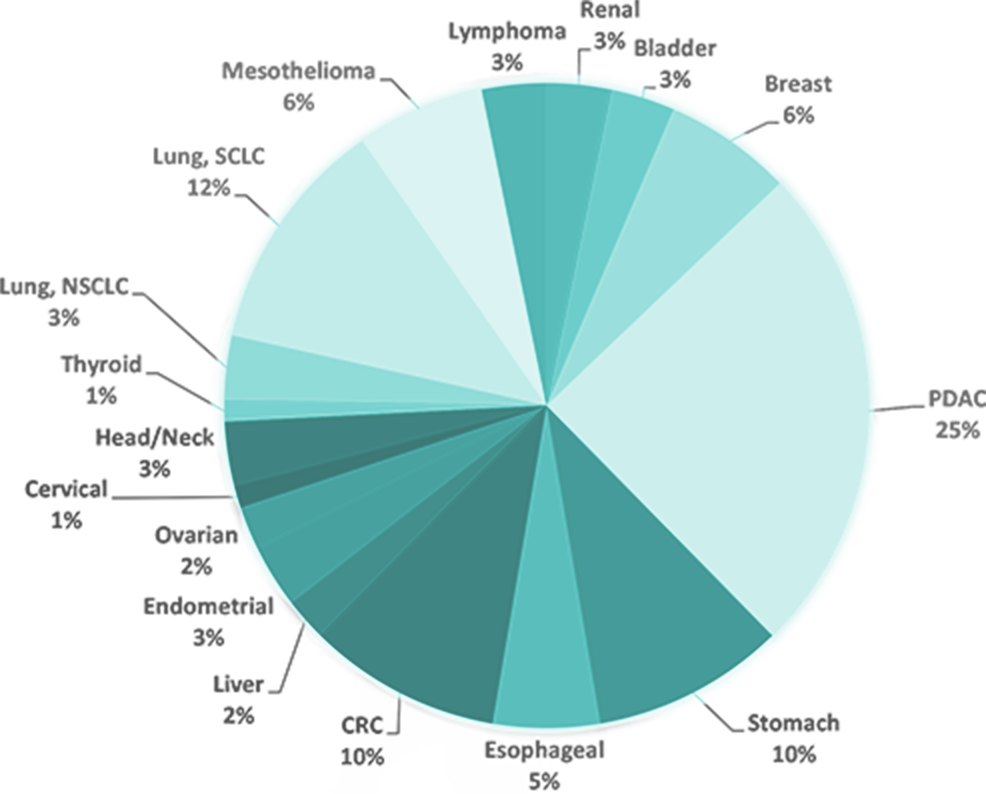
Our unique collections of patient-derived xenograft (PDX) models used to evaluate safety and efficacy include:
- Pancreatic Ductal Adenocarcinoma (PDAC) Pancreatic Cancer PDX Collection - in vivo screening for drug efficacy, tumor kinetics and animal survival studies
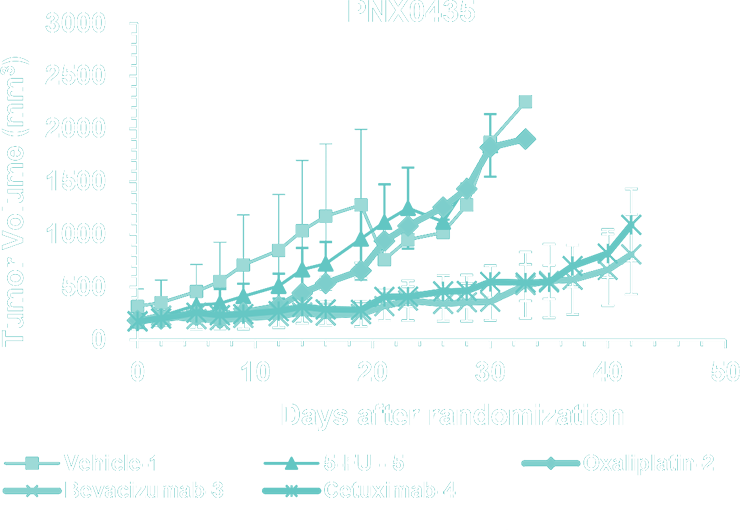
- Gastrointestinal (GI) PDX Collection (Esophageal, Gastric, Colorectal and liver cancer models) - histological analysis and SOC studies
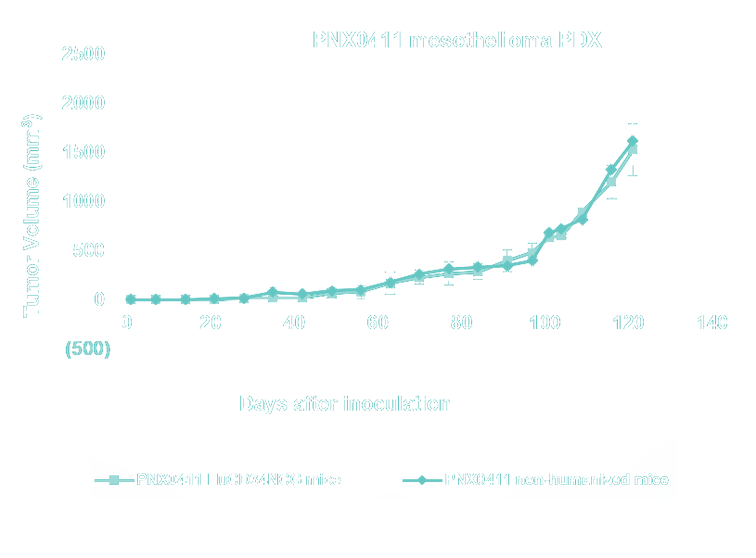
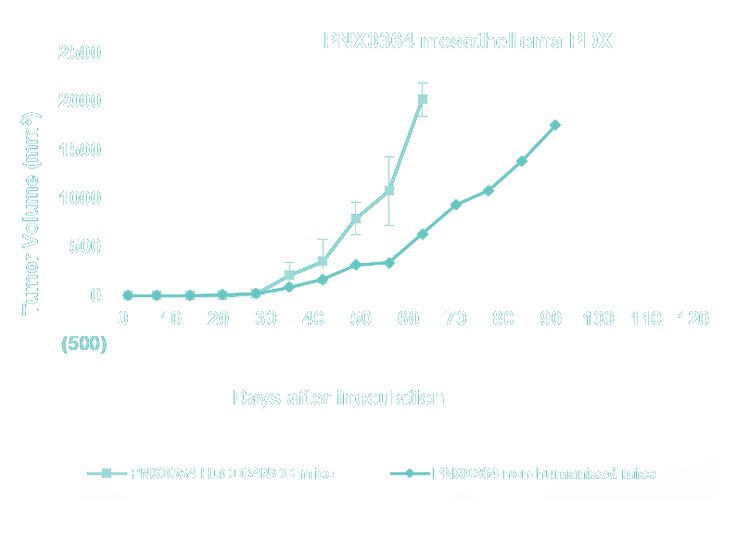
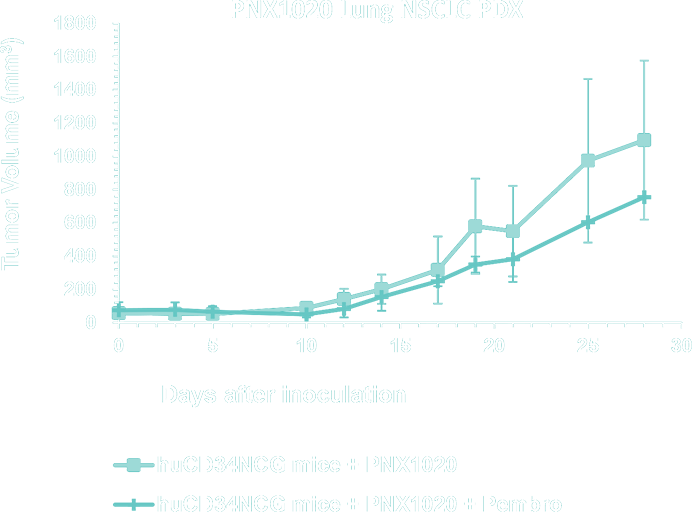
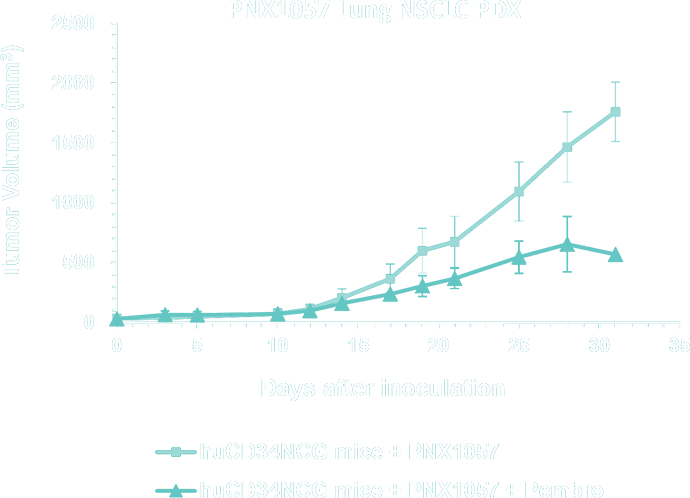
- Lung Cancer, Non-Small Cell Lung Cancer (NSCLC), Small Cell Lung cancer (SCLC) and Mesothelioma PDX Collection - WES - mutational and RNA-seq gene expression analysis for model selection
- Lymphoma PDX Collection – SOC studies, subcutaneous (SQ) and disseminating implantation studies
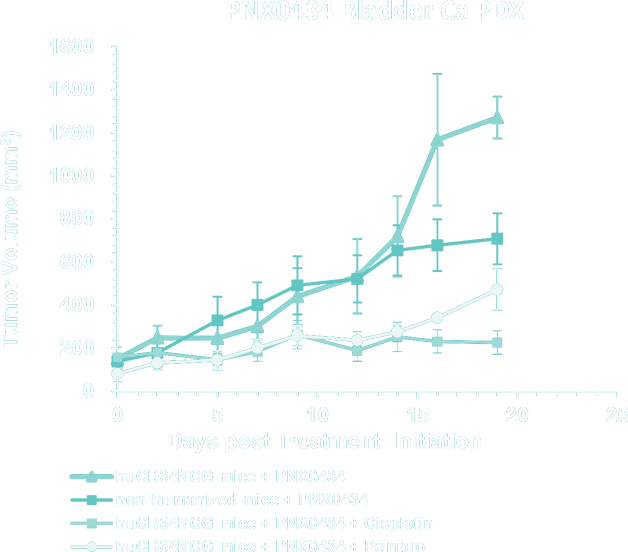
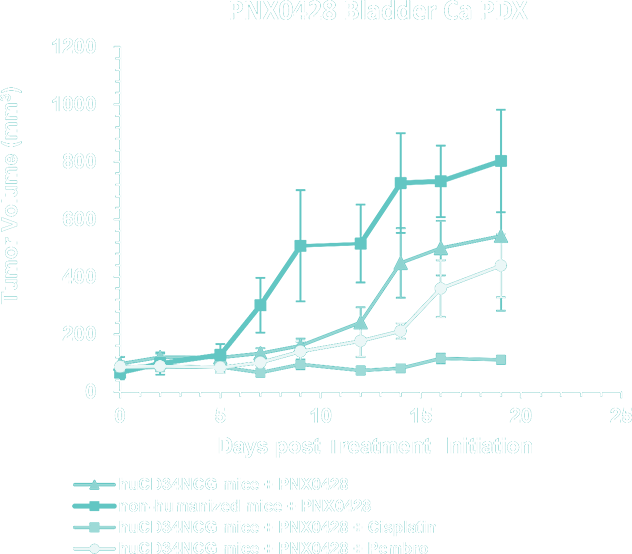
- Bladder Cancer PDX Collection – application of PDX models for immune-oncology
- Radiation Therapy Core –RT/drug combination studies with combinations of chemotherapies, targeted agents, and immune-oncology drugs with whole body and focal radiation therapy
Custom Models
GD3 offers to create PDX models based on the specific customer request. These models might be developed on exclusive and nonexclusive basis from primary and metastatic lesions with regard to patient ethnicity, medical history and treatment protocols.
PDX Discovery Platform
PDX models with established KRAS gene mutations
| PNX Model # | Tumor Type | Gene Name | Protein Change | DNA Change |
|---|---|---|---|---|
| PNX0079 | Endometrium | KRAS | p.G12V | c.35G>T |
| PNX0128 | Endometrium | KRAS | p.Q61H | c.183A>C |
| PNX0285 | Gastric | KRAS | p.G12D | c.35G>A |
| PNX0314 | Rectum | KRAS | p.Q61H | c.183A>T |
| PNX0314 | Rectum | KRAS | p.G13C | c.37G>T |
| PNX0419 | Colon | KRAS | p.G13D | c.38G>A |
| PNX0435 | Colon | KRAS | p.G12D | c.35G>A |
| PNX0001 | Pancreatic | KRAS | p.G12V | c.35G>T |
| PNX0017 | Pancreatic | KRAS | p.G12D | c.35G>A |
| PNX0045 | Pancreatic | KRAS | p.G12D | c.35G>A |
| PNX0050 | Pancreatic | KRAS | p.Q61H | c.183A>C |
| PNX0050 | Pancreatic | KRAS | T58I | c.173C>T |
| PNX0055 | Pancreatic | KRAS | p.G12D | c.35G>A |
| PNX0059 | Pancreatic | KRAS | p.G12D | c.35G>A |
| PNX0060 | Pancreatic | KRAS | p.G12D | c.35G>A |
| PNX0159 | Pancreatic | KRAS | p.G12D | c.35G>A |
| PNX0167 | Pancreatic | KRAS | G12R | c.34G>C |
| PNX0174 | Pancreatic | KRAS | p.G12D | c.35G>A |
| PNX0175 | Pancreatic | KRAS | G12R | c.34G>C |
| PNX0195 | Pancreatic | KRAS | p.G12V | c.35G>T |
| PNX0197 | Pancreatic | KRAS | p.G12D | c.35G>A |
| PNX0230 | Pancreatic | KRAS | G12R | c.34G>C |
| PNX0278 | Pancreatic | KRAS | p.G12D | c.35G>A |
| PNX0296 | Pancreatic | KRAS | p.G12D | c.35G>A |
| PNX0302 | Pancreatic | KRAS | p.G12D | c.35G>A |
| PNX0362 | Pancreatic | KRAS | p.G12V | c.35G>T |
-

IRB 12-822 -

Patient-derived xenografts (PDX) -
Xenopatient Trials of New Drugs -
Active Drugs -

Validation in GEM -
Path to Clinical Trials
Examples of Study Services
Genomic Analysis Of PDX Models
ANTRX1 Gene Expression In a Subset of PDX Models
Relative mRNA Expression of ANTRXR1 in PDX Tumors
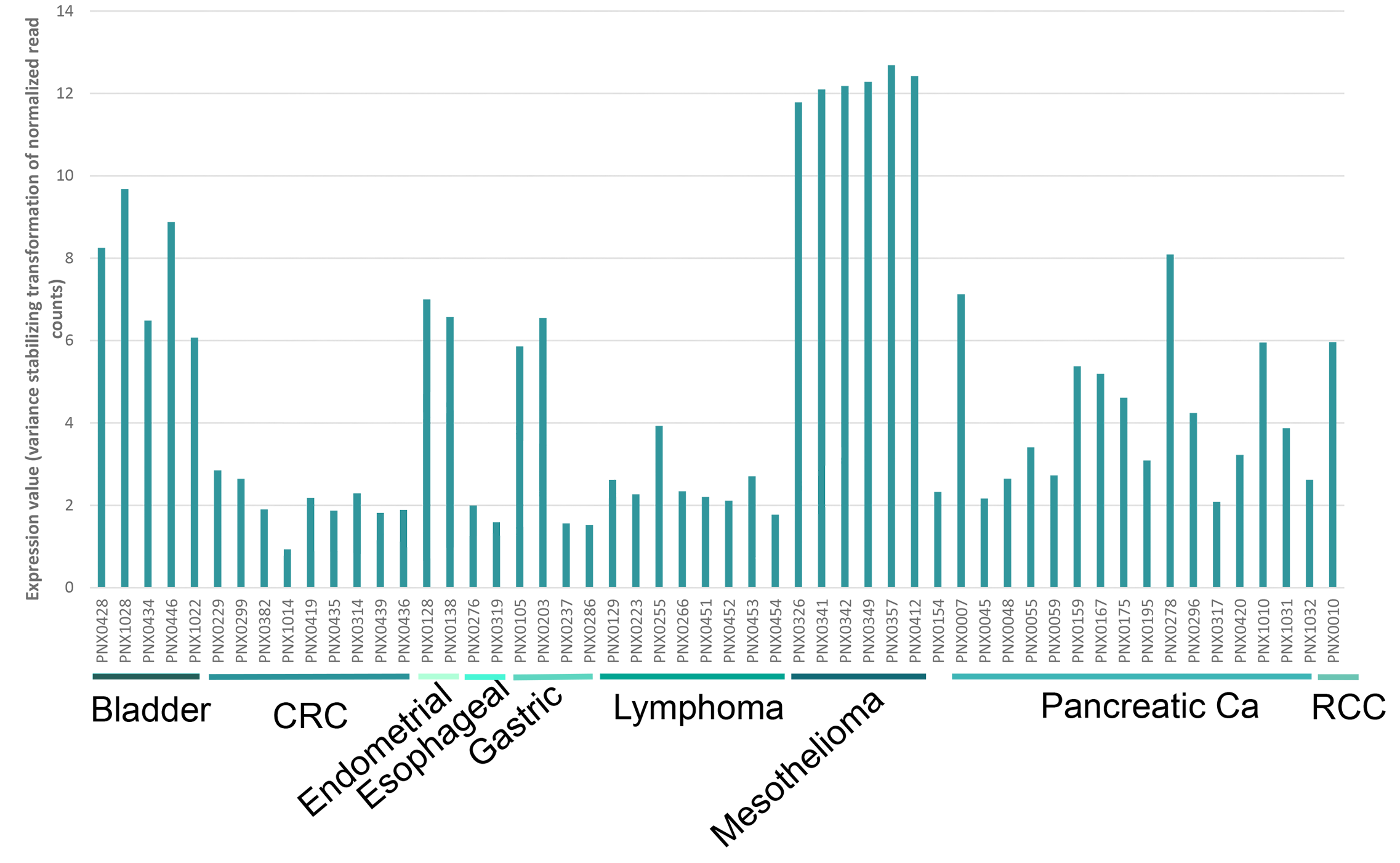
ANTRX1 Gene Expression in Various Tumor Types
mRNA Expression of ANTRX1 in Various Tumor Types
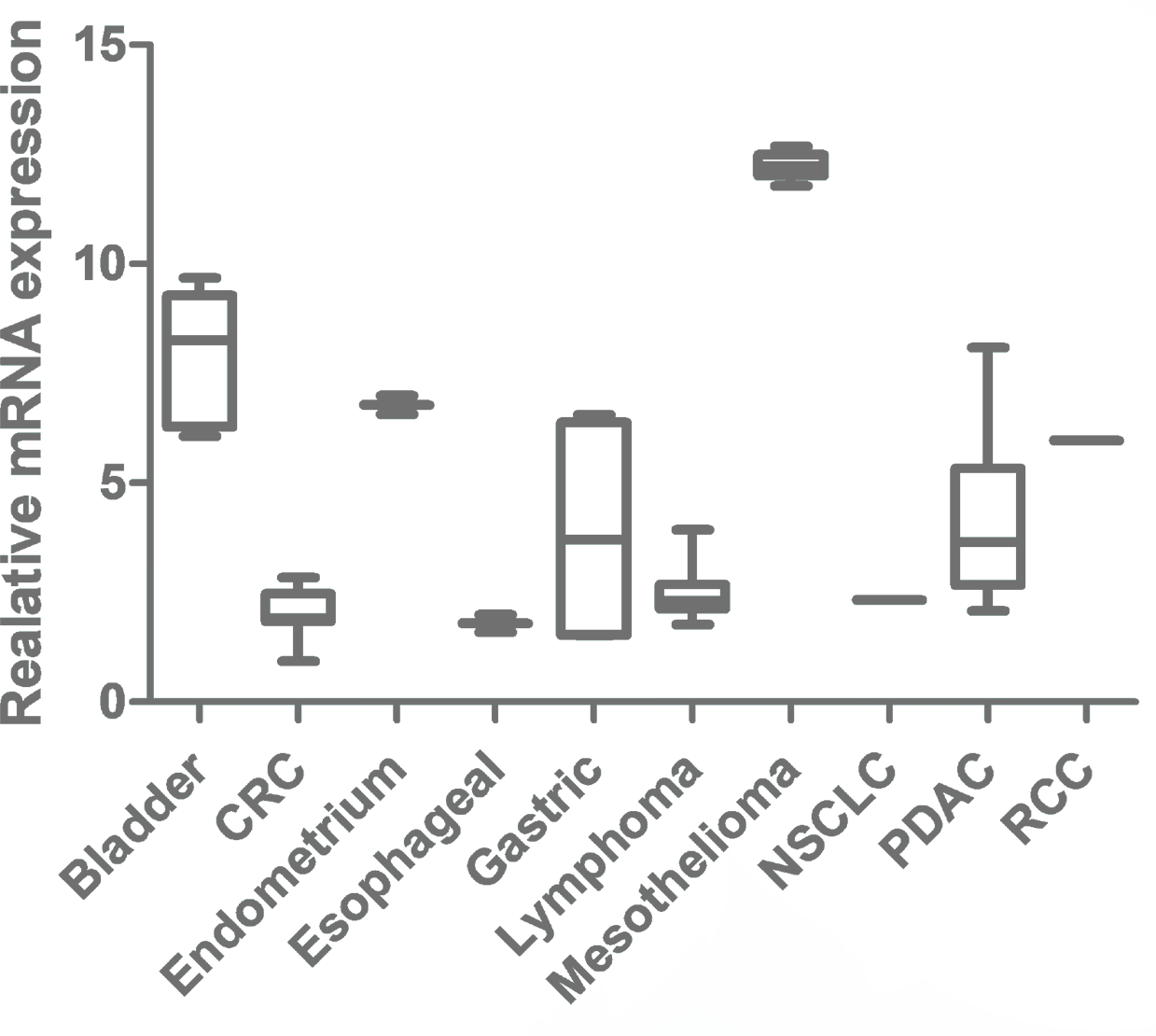
Data Analysis
Validation with SOC
(for selected model)

Molecular Characterization
- IHC
- Targeted gene expression profiling
- Gene Mutation analysis by WES and NGS gene panels
Patient Data