Biochemistry & Bioanalytical
Immunogenicity Assays
At GD3, we're committed to ensuring the safety and efficacy of biologic therapies. Our team of expert scientists specializes in conducting rigorous immunogenicity assays, meticulously evaluating the immune response elicited by these groundbreaking treatments.
From gene therapy to other cutting-edge biologics, we provide comprehensive services tailored to your specific needs. Our in-depth analysis helps identify potential risks early in the development process, enabling you to make informed decisions and bring safe, effective products to market.
-
Immunogenicity assays include:
- Screening assays
- Confirmatory assays
- Titration assays
- Neutralizing antibody (NAB) assay
- Regulated (FDA GLP) and non-regulated
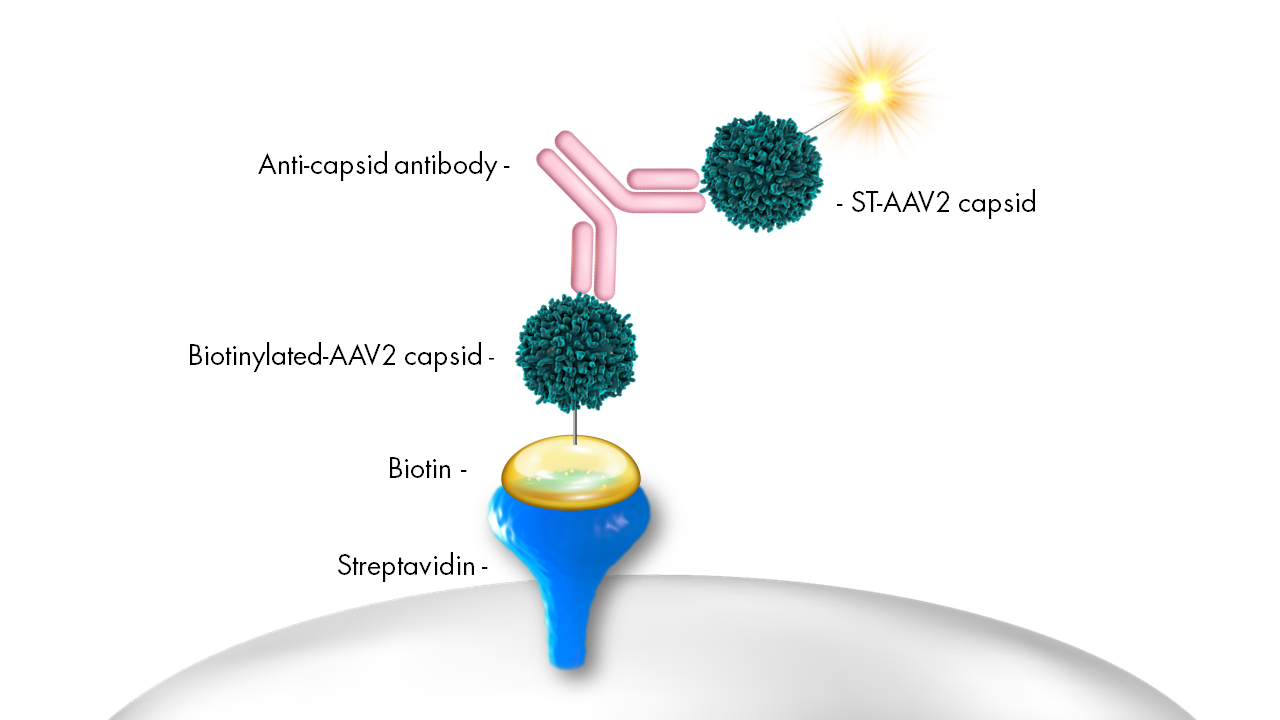
Figure 1. Sequential bridging immunogenicity assay capable of detecting anti-AAV2 antibodies.
Learn more about






























